How a Kitchen Experiment Spawned a New Science: The Surface Physics of Agnes Pockels.
- Dale DeBakcsy
- Oct 16, 2023
- 6 min read
In 1932, Irving Langmuir won the Nobel Prize for his life of work investigating the physics of how surfaces interact with their surroundings. In his Nobel lecture, he magisterially surveyed not only his own contributions to the still relatively young field, but those of other scientists including William Gibbs, James Dewar, W.D. Harkins, J. Traube, and H.S. Taylor. Palpably absent, however, was any mention of the woman whose work in the late 1870s and 1880s provided the standard tools by which liquid surfaces are prepared for observation, impurities introduced, and resultant properties measured, Agnes Pockels (1862-1935).
The omission of Pockels’s name from the pantheon of surface researchers by a figure whose work stood very much on the shoulders of her own trailblazing efforts was all the more hurtful because Pockels was still quite alive at the time of Langmuir’s speech, living in retirement and doing her best with the resources at her disposal to keep herself and her loved ones afloat during the Depression gripping Germany at the time. For Pockels, this was simply another low among the peaks and valleys that had defined her life. Had she been born a century later, her intellectual gifts would have been recognized and fostered from an early age, but for this daughter of a Royal Austrian Army soldier, born in 1862 in Venice but living most of her life in Brunswick, Lower Saxony, her scientific career had to be carved out from the thin slices of time allowed her by familial expectations and government policies.
Though avidly interested in science from an early age, the highest formal education open to young Pockels was high school, German universities not regularly allowing women students to attend as undergraduates until the 1890s (with a couple of extraordinary exceptions, such as Sofia Kovalevskaya’s receiving a degree from Göttingen in 1874). Added to the institutional roadblocks in the way of her exploring her interest in science beyond high school were societal ones, particularly the expectation that unmarried daughters would devote themselves in adulthood primarily to the care of their parents. As such, while her younger brother Fritz was allowed to attend the University of Göttingen and there receive his degree in physics, Agnes remained at home, tending to her parents’ needs for the next three decades.

Fortunately, Fritz took pity on his older sister’s state, and brought his Göttingen textbooks back home for her to teach herself physics and chemistry, though she claimed in an autobiographical sketch that she could never teach herself enough advanced mathematics purely from the books to go into the more theoretical aspects of those fields. What she did understand, however, allowed her to see the world in a way that most German caregiving daughters of the era did not, including finding inspiration for a scientific revolution in the most unlikely of places: a sink of dirty dishwater.
For generations, women had been looking down at soapy dishwater, but it took Pockels’s unique mind to see in that shimmering mass an interesting chemico-physical problem that required investigation. She wondered how the addition of impurities like soap to water affected its chemical properties, and how she could, with just the materials at her disposal, design an experimental apparatus to investigate those properties. She began her experiments at age 18 and by age 20 she had largely perfected the apparatus that would become a standard tool of surface chemists and physicists to the present day, which by a cruel twist of fate is known as a Langmuir Trough, named after the Nobel laureate we met above who modified the device to its modern form and who neglected to mention Pockels’s significance in originating his trademark scientific tool even once in the thirty seven pages of his Nobel lecture.
Pockels’s trough is a really neat thing, and worth talking about in some detail. Essentially, it is composed of a trough of water filled up to the edge, with a piece of tin that can be placed on the very top and slid back and forth, and a disc (originally a simple ceramic button) attached to a thread that is itself attached to an arm with a sliding weight that can be used to raise and lower the disc onto the water’s surface. Pockels would begin by sliding the tinfoil across the water surface, scraping off any impurities as it passed by. She would then add susbstances such as oil to the pure surface, and measure the change in the water’s surface tension by recording how much weight it took to pull the floating ceramic disc off the surface. She soon found that the addition of impurities significantly lowered the surface tension of water, and developed a further step of sliding the tin strip back to the left, progressively bunching up the surface impurities, measuring the change in the tension all the while, discovering that increased impurity density reliably resulted in decreased surface tension, up to a point.
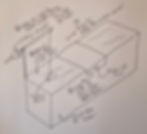
That point, which now bears her name, is the Pockels Point. This is the point when
the impurity particles are shoved so close together that they start repelling each other, and can’t be made to pack any closer. Pockels noted that, once that point is reached, no matter how much more she moved her tin strip to the left, surface tension remained the same. These results, along with the methods she developed for introducing monomolecular layers of impurities of different molecular types to the water surface, represented the cutting edge of her era’s investigations of surface properties, but at first scraped up but little interest among German scientists. It was not until 1888, when she read some abstracts of similar (but far less advanced) work by the great British scientist Lord Rayleigh (1842-1919), and was prodded by her brother to send her results to the eminent man, that a member of the official scientific establishment took active interest in her investigations. Rayleigh was so taken by her experiments that he translated them himself and had them published in Nature in 1891, with an introductory note of his own lauding the results she had obtained and questions she had opened that were beyond those he had managed on his own.
Her results publicly praised by the second Cavendish Professor of Physics himself, Pockels found herself suddenly being courted by the German scientific community. Crystal physicist Woldemar Voigt (1850-1919) offered her lab facilities at the Physical Institute, and for a little over a decade she was able to continue her work in and around her responsibilities at home, including investigations into how water impurities affect the ability of waves to propagate themselves.
By 1902, however, her parents’ health was in such a precipitous state of decline that caring for them became Pockels’s full time job, and she left behind her scientific work to tend to their needs. Her father passed away in 1906, her brother in 1913, and her mother in 1914. Though emotionally traumatic, the death of her immediate family might have allowed her to return to science, but with the arrival of World War I and Germany’s isolation from the world community, she found herself unable for years to access the world’s scientific literature. Subsequently, with Germany’s ultimate defeat in that conflict, though the lines of scientific communication were opening again, Pockels’s declining health and eyesight, and her sense of responsibility to use her time and resources (she was somewhat insulated from the effects of the post-war German economic depression from her wise investments in American companies) to help the people around her avoid the threat of starvation and homelessness, kept her from a return to her research.

Even as she was letting go of her old career, save for the occasional article refining and outlining her classic results, the German scientific community rediscovered her foundational work, and bestowed upon her the Laura Leonard Prize in 1931, an honorary doctorate from Carolina-Wilhelmina University in 1932, and a retrospective of her work by the legendary physical chemist Wilhelm Ostwald in 1932 (the year of his own death) in the pages of Kolloid-Zeitschrift, making up, one hopes, for the omission of her name from the Nobel ceremony centered around work that had grown from her own.
Agnes Pockels died in 1935, one of the greatest citizen scientists of hers, or any, age, whose ability to see an enigma at the center of a mundane tub of dishwater gave birth to a new science, and a new realization that, sometimes, probing beyond the surface of an object in search of deeper mysteries is to miss some of the most profound puzzles of all.
FURTHER READING:
Ostwald’s article, which contains Pockels’s autobiographical notes, is hard to track down, so for the most part you’re left with the accounts of her life contained in Out of the Shadows: Contributions of Twentieth Century Women to Physics, edited by Nina Byers and Gary Williams, and the ever-trusty Women in Chemistry and Physics: A Biobibliographic Sourcebook, edited by Grinstein, Rose, and Rafailovich.


